

Does Your Training Program Have Traction?
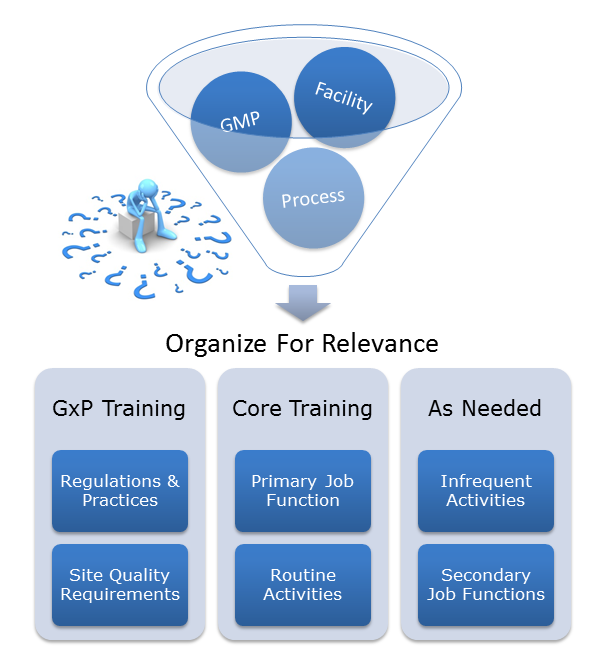
As companies scramble to get projects back on track and inspection-ready, one area that may be easily forgotten is employee training. According to the FDA, training should occur "on a continuing basis and with sufficient frequency to assure that employees remain familiar with GMP requirements applicable to them." To meet this requirement, maintaining a GMP workforce culture is critical.






